Abstract
ASXL1 plays a vital role in regulation of the histone methylation and acts as a tumor suppressor. Majority of ASXL1 mutations are frame-shift and nonsense mutations. The most frequent mutation is C.1934dupG (P.Gly646TrpfsX12) with a loss of PHD domain and truncated protein formed. Reduced normal ASXL1 protein expression promotes myeloid transformation. ASXL1 mutations change the transcription of certain oncogenes and result in dysfunction of tumor stem cells.
We found that ASXL1 mutations were identified in 29.1% (32/110) patients with primary myelofibrosis (PMF). Moreover, JAK2V617F mutated patients with ASXL1 mutations were more likely to present splenomegaly (median LCM: 10 vs. 5 cm, P=0.023), while in JAK2V617F negative patients, the association was gone. There is evidence that SDF-1α/CXCR4 axis plays an important role in the adhesion of leukemic cells and bone marrow stromal cells. Abnormal CXCR4 expression may contribute to constitutive migration of CD34+ cells in PMF. Accordingly, we supposed that on the basis of JAK2V617F mutation, mutated ASXL1 acts on the SDF-1α/CXCR4 axis leading to the stem cell mobilization and splenomagly.
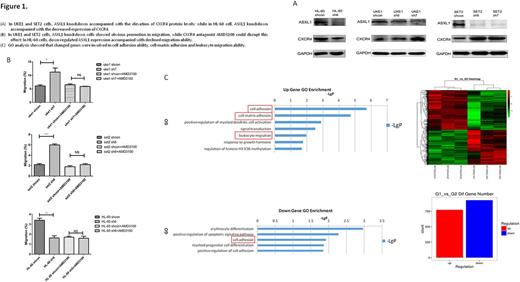
To further investigate the molecular mechanism of ASXL1 mutation in patients with JAK2V617F, we performed short hairpin RNA (shRNA)-mediated stable knockdown of ASXL1 in JAK2V617F mutated UKE1 and SET2 cell lines and JAK2V617F negative HL-60 cell line. In JAK2V617F mutated UKE1 and SET2 cells, ASXL1 knockdown accompanied with the significant elevation of CXCR4 mRNA and protein levels (Figure 1A); while in HL-60 cell, ASXL1 knockdown accompanied with the significant decreased expression of CXCR4 (Figure 1A). Next, we tested the effect of ASXL1 expression on cell migration ability by using transwell migration assay. Our observation showed that ASXL1 knockdown cells showed obvious promotion in migration in JAK2V617F mutated UKE1 and SET2 cells (P=0.028 and P<0.001 ), and CXCR4 antagonist AMD3100 could disrupt this effect (Figure 1B), and down regulation of ASXL1 expression accompanied with declined migration ability in HL-60 cells (P=0.0025, Figure 1B). Flow cytometry also confirmed the increased membrane CXCR4 expression in JAK2V617F mutated UKE1 and SET2 cells. In order to further explore the mechanism of ASXL1 effect on cell migration, we performed mRNA expression microarrary and the results showed that the function of cell adhesion ability, cell-matrix adhesion and leukocyte migration ability were up regulated in ASXL1 knockdown SET2 cells (Figure 1C).
In summary, our data demonstrated that ASXL1 knockdown in JAK2V617F mutated UKE1 and SET2 cells resulted in increased expression of CXCR4 and enhanced cell migration abilities, which may indicated that ASXL1 mutation may act on SDF-1/CXCR4 axis. We speculate that under the JAK2V617F mutated background, ASXL1 knockdown may promote the migration ability of tumor stem cells and formation of tumor microenvironment through acting on SDF-1/CXCR4 axis and result in splenomegaly and extramedullary hematopoiesis in PMF patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal